Adhesion is the physical state of an interface layer that is between two condensed phases that come into contact, i.e. H. Solids and liquids with negligible vapor pressure. The state is characterized by molecular interactions in the interface layer, which cause mechanical cohesion of the phases involved. In most cases, the cohesion between the materials involved is based on physical interactions. If the surface is sufficiently rough, mechanical interlocking of the materials can also occur. If the materials also form a chemical bond with one another, a particularly strong and lasting connection is usually formed. Examples of the formation of chemical bonds between binders and certain materials are silicone and glass, polyurethane and wood or epoxy resin and aluminum. In physics and chemistry, cohesion refers to the binding forces between atoms and molecules within a substance. The forces ensure its cohesion. They work in gases, liquids or solids and lead to surface tension on the surfaces of a liquid substance. The capillary effect describes the behavior of liquids in capillaries, i.e. sufficiently narrow tubes, gaps or cavities with non-deformable surfaces made of solids. This is determined by the surface tension of the liquid (cohesion) and the interfacial tension between the liquid and the solid surface (adhesion) or the wettability of the solid surface with the liquid. Since the weight of the liquid is low in narrow cavities, capillary force outweighs gravity and helps trees, for example, allow water to rise up to 100 meters from the roots.
Rise height h in capillary:
h = 2*σ*cos(θ) / ρ*g*r
σ = surface tension
θ = contact angle
ρ = density of the liquid
g = gravitational acceleration
r = radius of the tube
For a water-filled glass tube open to air at sea level (1,013.25 hPa) is:
σ = 0.0728 J/m² at 20 °C
θ = 20° = 0.35 rad
ρ = 1000 kg/m³ g = 9.81 m/s²
Wetting: Is the formation of an interface between a liquid and a solid.
Wettability is the associated property of the solid surface. In the course of wetting processes, the contact area between the wetting liquid and the wetted solid surface increases until a static state is reached, which is characterized by the existence of a constant contact area. The extent of wetting depends on the type of liquid and the nature of the solid surface, such as its chemical composition and roughness. Wetting phenomena are relevant for coating, painting and printing surfaces, the distribution of herbicides and insecticides on agricultural land, filtration and dispersion. When one speaks of wetting, one describes the behavior of liquids when they come into contact with the surface of solids. The associated property of the surface of the solid is the so-called wettability. How much a liquid wets a surface depends on various factors such as type of liquid, material or surface or condition of the surface. Wetting is directly related to surface tension. If the cohesive force within a water drop is smaller than the adhesion force with respect to the surface of the solid, the drop spreads on the surface of the solid and it is completely wetted. On the other hand, if the cohesive force is greater than the adhesion force, the water drop takes on a spherical shape and the surface is hardly wetted. Many surfaces, even when clean, have insufficient wettability, which is exacerbated by contamination, which results in liquids rolling off adhesives, varnishes and paints.
This is because the surface tension is very low and not sufficient for further processing. When unprocessed material is processed, the result is often that paint and varnish do not adhere properly and come off quickly or that bonded parts fall apart. This can be prevented with plasma activation. The plasma activation of a surface increases its surface energy and creates deposit points for the applied liquid. This means it can adhere very well. Plasma activation modifies the surface and builds up surface energy, resulting in significantly better surface wettability.
The smaller the contact angle or surface tension, the greater the wetting tendency.
Surface tension is the phenomenon that occurs in liquids as a result of molecular forces to keep their surface small. This effect, for example, is what causes water to form droplets and contributes to the ability of some insects to walk on water and the ability of a razor blade to “float” on water. The surface tension (formula symbol: σ, γ) is an interfacial tension that occurs between liquids and gas phases. It is measured in the SI units N/m.
Surface tension of water at 20°C = 72.75 mN/m.
Mechanical definition: The mechanical definition can be explained using a bracket with width L in which a film of liquid is clamped. If the liquid film is pulled apart by dx by a force F parallel to the surface and perpendicular to L, work is done on the film dW = F*dx is done and the surface grows by dA = 2*L*dx (factor 2 because of the front and back of the film). The surface tension is the ratio γ = dW*dA = F*2*L . Accordingly, surface tension is a force per length that is directed parallel to the liquid surface. The correctness of the idea of surface tension as a force parallel to the surface is shown in numerous measurement methods and effects such as the ironing method, capillarity or the contact angle.
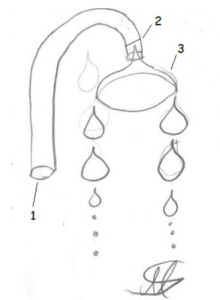
The wetting capillary (1) sucks up the water up to the rise height h (2), which depends on the diameter, and is curved downwards in the upper area. The wetting screen (3) is thinner at the tip than the capillary and the contact angle is very small, which means that the liquid spills over and drips off at the bottom of the screen.
Read more on https://www.nature.com/articles/s41598-019-57109-z
Which is also about an artificial tree (source scienceSketch):
Author:Markus Schrems