A research team at UC Berkeley has invented a catalyst that can breathe new life into waste plastic by turning it into adhesives with the same properties as the original material
Plastic pollution is one of the most difficult problems in the world today. One of the biggest obstacles is the inability to recycle certain types of polymers. This shortens the life of the material, lowers the value of the waste product and creates major environmental problems. The spread of multiple types of plastics is due to the wide range of properties that can be imparted to the end products. Variations in composition, different production methods and the use of additives create materials that are very different from each other and suitable for almost any industrial application.
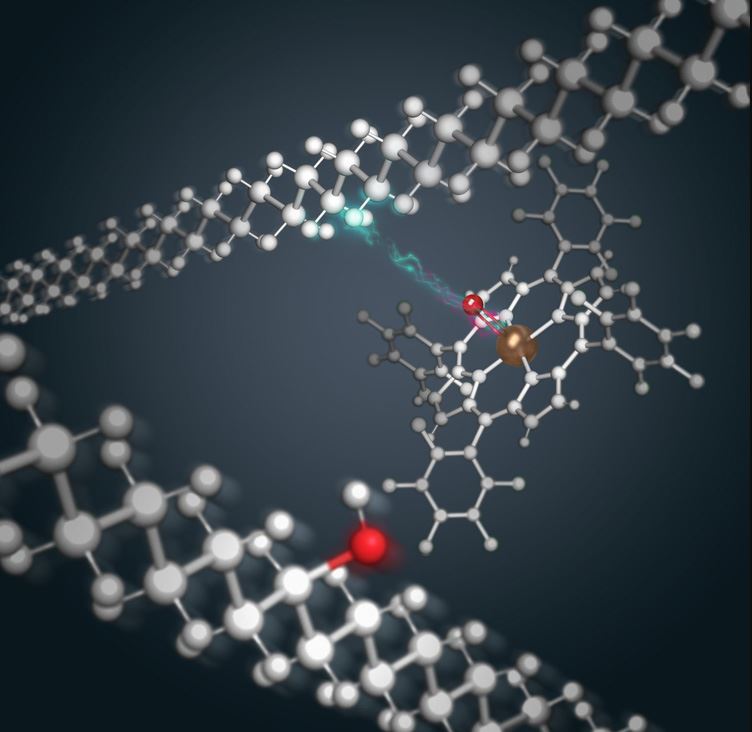
Among the types of plastic, polyethylene is the most common and its production exceeds 100 million tons worldwide each year. This polymer is mainly used in the production of packaging, bags, membranes, agricultural sheeting, toys and household utensils. Jonh Hartwig, who heads a research group at UC Berkeley, has developed a process to recycle polyethylene while retaining many of its original properties. The researchers added hydroxyl groups (oxygen and hydrogen) and produced an adhesive capable of adhering to metal through a ruthenium-based catalyst. The resulting adhesive can also be colored through water-based paints, which is impossible with the starting polyethylene. The results were published in the journal Chem.
“We are able to enhance adhesion, while preserving all the other traits of polyethylene that the industry finds so useful. The processability, thermal stability and mechanical properties seem to be unharmed while enhancing adhesion. That is tricky to do. That is really where we have some exciting things to show. Polyethylene usually has between 2,000 and 10,000 carbons in a chain, with two hydrogens on each carbon — really, it is an ocean of CH2 groups, called methylenes“, said co-author Phillip Messersmith, Professor in UC Berkeley’s departments of bioengineering and materials science and engineering. “We dipped into the literature to look for the most active catalyst we could find for functionalization of a methylene position.” “The catalysis introduced chemical changes to less than 10% of the polymer, yet enhanced dramatically its ability to adhere to other surfaces“.
You might also be interested into -> Here is the infinitely recyclable plastic